
|
Callus and adventitious roots
When sections of a stem or a hypocotyl of Ipomoea nil were cultured on an agar nutrient basic medium including plant hormones, a callus occurred and an adventitious root occurred easily. Root-like structures were produced from the bottom of the callus shown in the Figure. However, differentiation of adventitious buds was not found.

|
Decotylated embryo culture --> Callus and various forms of protuberances
After removing the cotyledons surgically from the embryo of a cotyledon stage one week after fertilization and culturing a hypocotyl including a radicle, a callus occurred from a cut end, and projections of various shapes (tubes, cups, bars) arose from the border region of a callus and a hypocotyl. With this technique, you don't need to add any particular hormone to agar nutrient medium.

|
Hypocotyl end culture --> adventitious embryo
When the hypocotyl tip of an embryo at the cotyledonary stage one week after fertilization was cultured, an adventitious embryo occurred together with a projection of various shapes. Projections developed on the hypocotyl tip as shown in this figure, and an adventitious embryo appeared on the tip.

|
Decotylated embryo culture --> regenerated plantlets developed from the adventitious embryo
In the regenerated plantlets that developed from an adventitious embryo, you can see a stem and a leaf.

|
Decotylated embryo culture --> a regenerated plantlet developed from the adventitious embryo
In the regenerated plantlets that developed from an adventitious embryo, you can see a stem, a leaf, and a root.

|
Decotylated embryo culture --> a regenerated plantlet developed from the adventitious embryo
The regenerated plantlets were transplanted into fairly big glasswares from test tubes.

|
Decotylated embryo culture --> transplant of the plant to a pot
The regenerated plantlets were transplanted into a flowerpot.

|
Decotylated embryo culture --> a regenerated plantlet developed from adventitious embryo
The results were shown when a strain derived from the Violet strain was cultured by similar methods. A leaf, a stem, and a root were differentiated in a regenerated plantlet developed from an adventitious embryo.

|
Decotylated embryo culture --> a regenerated plant
The regenerated plant transplanted to a glass bottle.

|
Decotylated embryo culture --> a regenerated plant
The regenerated plant that flowered in a glass bottle.

|
Decotylated embryo culture --> a regenerated plant
The regenerated plant transplanted into a flowerpot from a glass bottle.

|
Decotylated embryo culture --> a regenerated plant
Flowering of the regenerated plant that was transplanted into a flowerpot from a glass bottle.

|
Decotylated embryo culture --> a regenerated plant
A regenerated plant matured and opened double flowers.
|
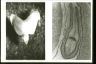
Young embryo (at cotyledonary stage) about one week after fertilization
The left figure shows the embryo at the cotyledonary stage taken out from an ovule of closely related species Ipomoea hederacea. The right figure shows the tissue structure of the embryo at the cotyledonary stage in the embryo sac of an ovule.

|
Decotylated embryo --> adventitious embryo formation
Cotyledon of the embryo at cotyledonary stage was surgically removed (this is called decotylated embryo). When this decotylated embryo was cultured, various forms of protuberances occurred.

|
Decotylated embryo culture --> a regenerated plantlet
A plantlet developed from an adventitious embryo.

|
Decotylated embryo culture --> a regenerated plantlet
The regenerated plantlet that was cultured and produced on nutrient basic medium with the addition of 55 mg/l glutaminic acid.

|
Decotylated embryo culture --> transplant of the regenerated plant
Plantlet transplanted to a flowerpot from glassware. The plantlet was covered with a beaker for a while to avoid a drastic change of environment.

|
Decotylated embryo culture --> a regenerated plant
The regenerated plant flowered in a flowerpot.

|
Decotylated embryo culture --> a regenerated plant
A further grown, flowering regenerated plant